The role of the medial prefrontal cortex (mPFC) in top-down executive control: a review of fear conditioning paradigms
Note: This essay was written during my Master’s degree for the course Methods in Behavioral Neuroscience. I got a 7.5 (B+) for it, so I thought I would share.
Abstract
The processes that underlie aversive memory formation have long been elusive, in part because of the complexity of the brain-wide, sparse network of cortical regions that influence fear behavior, which includes the amygdala, hippocampus and mPFC. The mPFC is thought to provide top-down control over behavioral output, but the specifics of the microcircuitry and role of specific neuronal subtypes that are involved in this process remain poorly understood. The goal of this mini-review paper is to obtain a better insight into the role of the mPFC in fear conditioning by providing an overview of papers that have used state-of-the-art technology and provide future perspectives in the field.
What is the PFC?
The prefrontal cortex is the association cortex of the frontal lobe (Fuster, 2001). The PFC is involved in working memory, attention and decision making, cognitive processes collectively referred to as “executive functions” (Domenech & Koechlin, 2015). These higher-level cognitive functions of the PFC derive from its unique anatomy – it has extensive afferent and efferent connections to many brain regions, including the hippocampus, amygdala, basal ganglia, motor and sensory cortices (Gabbott et al., 2005). The PFC is therefore thought to be the ‘goal-directed’ cortex because it exerts top-down control over limbic, sensory and motor information and allows the organism to perform the most optimal behavior to the specific environmental conditions while inhibiting alternative actions primed by these bottom-up systems (Kamigaki, 2019).
Much of our current understanding comes from animal models, particularly mice. It may initially seem counterintuitive that the research on the rodent PFC could be useful to humans, since this brain region comprises 30% of the human cortical area, it is the last brain region to mature in adults and has specific anatomical regions not analogous to any other mammal (Neubert et al., 2014). While it is true that the murine brain is much simpler and has significant anatomical differences to the human PFC, e.g. a lack the granular layer IV in the cortex, it is now widely accepted that the mouse PFC has functional similarities: they can learn tasks that require attention (e.g. 5CSRTT), working memory (e.g. delayed matching-to-position task) and decision making (e.g. delayed reward paradigm).
The rodent PFC is broadly divided into three regions: ventral, lateral and medial. The medial prefrontal cortex (mPFC) has been extensively studied and found to be associated with many processes, including executive functions, recent and long memory formation (Euston, Gruber, & McNaughton, 2012). The mPFC can be broadly subdivided into a dorsomedial portion (dmPFC) and a ventromedial portion (vmPFC) which are differently connected to other brain regions: the vmPFC is more highly connected to limbic structures while the dmPFC is more highly connected to sensory and motor regions. The dmPFC comprises the anterior cingulate (AC) and the dorsal-most part of the prelimbic cortex (PL). The vmPFC comprises the infralimbic cortex (IL) and the ventral-most part of the prelimbic cortex. Because there is no clear division between dmPFC and vmPFC, only a gradient of projections to cortical and limbic structures in the PL (Gabbott et al., 2005), this review will mention the specific mPFC subregion whenever possible.
The goal-orienting nature of the mPFC is evolutionarily important because of the unpredictability of nature – while instincts can be developed for intrinsically stereotypical cues, much of the environment is highly context-dependent, and the organism must learn over its lifetime good or bad associations between environmental stimuli, behavior and outcome results. Fear conditioning has been extensively used to study long-term aversive memory and how sensory input ultimately generate adaptive behavior (Herry & Johansen, 2014). This paradigm has many advantages over other aversive memory paradigms, principally because it allows for a minimal amount of training – one session with a duration of a few minutes will promote permanent memory changes.
The goal of this mini-review paper is to obtain a better insight into the role of the mPFC in the orchestration of behavior in response to aversive stimuli, addressing current controversies in the published literature and indicating potential future research directions that could address the current gaps in knowledge.
The role of the mPFC in fear memory
Fear conditioning involves the association of a neutral stimulus contingently paired with an aversive stimulus. In this paradigm, a conditioned stimulus (e.g. an auditory tone) is paired with an unconditioned stimulus (e.g. a foot shock). The conditioned auditory stimulus and unconditioned shock stimulus enter the amygdala at the level of the basal and lateral nuclei of the amygdala (collectively known as BLA), where they are biologically paired. The BLA is connected to the central nucleus (CeA), which is the major output system of the amygdala, which then promotes the conditioned response. The neuronal connections that underlie a memory are named memory engrams (Josselyn, 2010). The level of fear is measured from the stereotypical behavioral output – i.e. how much time the animal spends on the freezing state (Jacobs, Cushman, & Fanselow, 2010), and manipulations in the memory engram can be performed with techniques such as optogenetics, chemogenetics or activity-dependent labeling.
The mPFC is highly interconnected with the amygdala, mainly with the BLA. The IL is thought to mediate extinction by increasing the activity of GABAergic intercalated cells, which decreases the activity of the CeA. The PL is thought to increase the activity of glutamatergic neurons in the BLA, increasing the output of the CeA and promoting the fear response (Marek, Sun, & Sah, 2019). However, the PL and IL are not as independent as this simplified model might suggest. There are glutamatergic neurons that project from PL to IL, and specific excitation of these enhances fear extinction (Marek et al., 2018). Davis et al. (2017) showed that chemogenetic inhibition of BLA parvalbumin-positive (PV) interneurons lead to an increase in freezing behavior after extinction learning. Using active dependent labeling, the authors found that this disinhibition in the BLA leads to the reactivation of engram neurons in the mPFC – indicating a functional role of BLA PV interneurons in extinction. In the same study, the authors found that the IL had more projections to BLA PV interneurons compared to PL projections and the BLA had more projections to the PL – establishing a reciprocal loop between mPFC and BLA.
The process of extinction, i.e. weakening the association between CS-US upon repeated exposures of the same context without the shock, is mediated by the IL. Extinction does not erase the original fear memory, but rather creates a new memory (An et al., 2017). Extinction learning can be enhanced by optogenetically stimulating the IL and impaired by chemogenetically silencing this brain region (Bloodgood et al., 2018). This leads to an interesting question of how output behavior could be mediated with two contradictory fear memories. Davis et al. (2017) used in vivo electrophysiology to record monosynaptic BLA PV interneurons projecting to the mPFC and found that fear conditioning leads to an increase in the 3-6 Hz range, silencing BLA PV interneurons decreased the 6-12 Hz range and the ratio of these two frequencies in the mPFC was correlated with the amount of freezing after extinction, but not before. This shift in network activity may be the neuronal correlate of the competition between fear memory and extinction memory.
The mPFC is also highly interconnected to the hippocampus, which is thought to provide contextual information about the fear memory. Rajasethupathy et al. (2015)used optogenetics to stimulate monosynaptic projections from the hippocampus to the AC after fear conditioning and found that mice would increase freezing behavior in an unrelated context. This effect was thought to be specific to the fear memory because, after extinction, the activation of this pathway no longer increased fear behavior. After placing the mice in a new context, the fear memory was reinstated, and the optogenetic activation again increased the fear memory. Kitamura et al. (2017) used a combination of activity-dependent cell-labeling, optogenetics and miniscopes to assess the activity of projections from the hippocampus to the PFC related to the fear memory. Inhibition of the engram had no significant effects on early fear memory but disrupted remote fear memory. The engram neurons in the mPFC were active in the original context in which the animals had received the shock 13 days after the original fear conditioning, but were silent 2 days later – i.e. the memory was not retrievable by environmental cues but could be activated with optogenetics. On the other hand, the hippocampal engram was active 2 days after fear conditioning, but silent 13 days later. The same authors hypothesize that the fear memory is initially encoded simultaneously in the mPFC, amygdala and hippocampus, but the engram undergoes maturation at different time points in the mPFC and hippocampus (Figure 1).
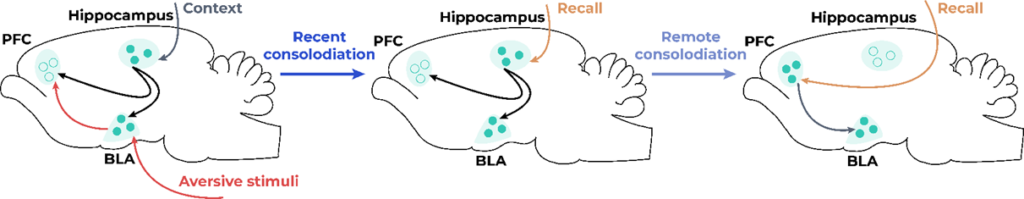
Contradictions in current findings
The mPFC has a great degree of complexity and interconnection to other brain regions: it comprises around 90% glutamatergic neurons, 10% GABAergic neurons and receives projections from several neuromodulator centers (serotonin from Ralphe nucleus, dopamine from the VTA, acetylcholine from the basal forebrain and noradrenaline from the locus coeruleus) (Dembrow & Johnston, 2014). Therefore, a unitary function for the mPFC cannot be expected – the role of this brain region will vary depending on specifics of the task at hand, sensory inputs, previous memory associations and internal needs of the animal. For example, the inactivation of PL has been found to have a number of contradictory effects in aversively motivated procedures – increasing fear memory, decreasing fear memory or having no effect at all (Sharpe & Killcross, 2018). The authors attribute those differences to differences in methodology – for instance, the duration of training and intertraining intervals, which may alter the learning association of the context with the aversive stimuli. Inactivation of the mPFC has been found to have no effect on acquisition, consolidation or retention of fear memory using Pavlovian condition, but it was required for acquisition, consolidation and retention when these three aspects of learning were temporally spaced (Heroux et al., 2017).
These contradictory effects could also be explained by the variability in engram targeting. When inactivating a brain region with chemogenetics/optogenetics, it is possible that an engram could or could not be targeted. The advance of more precise techniques for neuronal manipulation allows for the unraveling of the exact role that each cell type plays in the fear engram. For instance, Adhikari et al. (2015) found that optogenetically inhibiting projections from the vmPFC to the amygdala decreased the fear memory, while inhibiting dmPFC-ITC projections showed a decrease in fear behavior only in cued fear-extinction. The authors found that inactivating this specific vmPFC population leads to the suppression of fear memory, but not bulk activation of the entire vmPFC – which suggests a high variance of cell types in this region that may counteract the effects of the vmPFC-amygdala pathway. It is therefore of extreme importance to understand the mechanisms that make the memory engram active or silenced. Some of the possible mechanisms are: epigenetic changes in histone subunit (Zovkic et al., 2014), upregulation of AMPA receptors in engram neurons (Arruda-Carvalho & Clem, 2014), ratio of oscillatory activity between mPFC and BLA in the 3-6 Hz range over the 6-12 Hz range (Davis et al., 2017).
To conclude, the mPFC plays an important role in fear memory – remaining as a silent engram during early consolidation but becoming involved after late consolidation. Engram and cell-specific studies have been shown to be of incredible value, allowing for the modulation of neurons related to the substrate of fear memories and answering important questions raised by contradictory research done in the past. Current important questions remain: how are the neuronal substrates different for different types of memory (e.g. episodic/semantic)? How much of this knowledge applies to paradigms of different valences (either less stressful aversive memories or even positive memory paradigms)? And finally, how much of this knowledge is transferable to humans, based not only on the anatomical differences between humans and rodents but also the ecological validity of these paradigms? Future research could benefit from the use of more complex paradigms that use both aversive and appetitive stimuli depending on different cues and using miniscopes to assess how the engrams are differently formed and how much they overlap. The advance of the experimental toolbox for other mammals would also be of extreme importance since most of the current research on this topic uses mice and some of the findings could be species-specific. Replicating these findings using cell-specific and engram targeting in non-human primates would be of extreme relevance for the translational aspect of memory research.
References
Adhikari, A., Lerner, T. N., Finkelstein, J., Pak, S., Jennings, J. H., Davidson, T. J., … Deisseroth, K. (2015). Basomedial amygdala mediates top-down control of anxiety and fear. Nature, 527(7577), 179–185. https://doi.org/10.1038/nature15698
An, B., Kim, J., Park, K., Lee, S., Song, S., & Choi, S. (2017). Amount of fear extinction changes its underlying mechanisms. ELife, 6. https://doi.org/10.7554/eLife.25224
Arruda-Carvalho, M., & Clem, R. L. (2014). Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding. Journal of Neuroscience, 34(47), 15601–15609. https://doi.org/10.1523/JNEUROSCI.2664-14.2014
Bloodgood, D. W., Sugam, J. A., Holmes, A., & Kash, T. L. (2018). Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Translational Psychiatry, 8(1), 60. https://doi.org/10.1038/s41398-018-0106-x
Davis, P., Zaki, Y., Maguire, J., & Reijmers, L. G. (2017). Cellular and oscillatory substrates of fear extinction learning. Nature Neuroscience, 20(11), 1624–1633. https://doi.org/10.1038/nn.4651
Dembrow, N., & Johnston, D. (2014). Subcircuit-specific neuromodulation in the prefrontal cortex. Frontiers in Neural Circuits, 8(JUNE), 1–9. https://doi.org/10.3389/fncir.2014.00054
Domenech, P., & Koechlin, E. (2015). Executive control and decision-making in the prefrontal cortex. Current Opinion in Behavioral Sciences, 1, 101–106. https://doi.org/10.1016/j.cobeha.2014.10.007
Euston, D. R., Gruber, A. J., & McNaughton, B. L. (2012). The Role of Medial Prefrontal Cortex in Memory and Decision Making. Neuron, 76(6), 1057–1070. https://doi.org/10.1016/j.neuron.2012.12.002
Fuster, J. M. (2001). The prefrontal cortex – An update: Time is of the essence. Neuron (Vol. 30). https://doi.org/10.1016/S0896-6273(01)00285-9
Gabbott, P. L. A., Warner, T. A., Jays, P. R. L., Salway, P., & Busby, S. J. (2005). Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology, 492(2), 145–177. https://doi.org/10.1002/cne.20738
Heroux, N. A., Robinson-Drummer, P. A., Sanders, H. R., Rosen, J. B., & Stanton, M. E. (2017). Differential involvement of the medial prefrontal cortex across variants of contextual fear conditioning. Learning and Memory, 24(8), 322–330. https://doi.org/10.1101/lm.045286.117
Herry, C., & Johansen, J. P. (2014). Encoding of fear learning and memory in distributed neuronal circuits. Nature Neuroscience, 17(12), 1644–1654. https://doi.org/10.1038/nn.3869
Jacobs, N. S., Cushman, J. D., & Fanselow, M. S. (2010). The accurate measurement of fear memory in Pavlovian conditioning: Resolving the baseline issue. Journal of Neuroscience Methods, 190(2), 235–239. https://doi.org/10.1016/j.jneumeth.2010.04.029
Josselyn, S. A. (2010). Continuing the search for the engram: Examining the mechanism of fear memories. Journal of Psychiatry and Neuroscience, 35(4), 221–228. https://doi.org/10.1503/jpn.100015
Kamigaki, T. (2019, March 1). Prefrontal circuit organization for executive control. Neuroscience Research. Elsevier Ireland Ltd. https://doi.org/10.1016/j.neures.2018.08.017
Kitamura, T., Ogawa, S. K., Roy, D. S., Okuyama, T., Morrissey, M. D., Smith, L. M., … Tonegawa, S. (2017). Engrams and circuits crucial for systems consolidation of a memory. Science, 356(6333), 73–78. https://doi.org/10.1126/science.aam6808
Marek, R., Sun, Y., & Sah, P. (2019). Neural circuits for a top-down control of fear and extinction. Psychopharmacology, 236(1), 313–320. https://doi.org/10.1007/s00213-018-5033-2
Marek, R., Xu, L., Sullivan, R. K. P., & Sah, P. (2018). Excitatory connections between the prelimbic and infralimbic medial prefrontal cortex show a role for the prelimbic cortex in fear extinction. Nature Neuroscience, 21(5), 654–658. https://doi.org/10.1038/s41593-018-0137-x
Neubert, F. X., Mars, R. B., Thomas, A. G., Sallet, J., & Rushworth, M. F. S. (2014). Comparison of Human Ventral Frontal Cortex Areas for Cognitive Control and Language with Areas in Monkey Frontal Cortex. Neuron, 81(3), 700–713. https://doi.org/10.1016/j.neuron.2013.11.012
Rajasethupathy, P., Sankaran, S., Marshel, J. H., Kim, C. K., Ferenczi, E., Lee, S. Y., … Deisseroth, K. (2015). Projections from neocortex mediate top-down control of memory retrieval. Nature, 526(7575), 653–659. https://doi.org/10.1038/nature15389
Sharpe, M. J., & Killcross, S. (2018). Modulation of attention and action in the medial prefrontal cortex of rats. Psychological Review, 125(5), 822–843. https://doi.org/10.1037/rev0000118
Tonegawa, S., Morrissey, M. D., & Kitamura, T. (2018). The role of engram cells in the systems consolidation of memory. Nature Reviews Neuroscience, 19(8), 485–498. https://doi.org/10.1038/s41583-018-0031-2
Zovkic, I. B., Paulukaitis, B. S., Day, J. J., Etikala, D. M., & Sweatt, J. D. (2014). Histone H2A.Z subunit exchange controls consolidation of recent and remote memory. Nature, 515(7528), 582–586. https://doi.org/10.1038/nature13707