Alterations in vmPFC and hippocampus during development of depressive symptoms in non-human primate models
Note: This project proposal was written during my Master’s degree for the course Systems Neuroscience. I got a 8.0 (A) for this essay, so I thought I would share.
Background and Aim
Major Depressive Disorder (MDD) is the one of most common neuropsychiatric disorder, with a lifetime prevalence of 14.6% worldwide and 17.9% in the Netherlands1. MDD is a highly debilitating condition, it has a high economic burden (e.g. around 53 billion dollars in the US alone)2 and yet very little is known about its underlying neurobiology – current hypothesis range from inflammation3, mitochondrial dysfunction4, HPA axis dysregulation5 and gut-brain axis problems6. MDD presents many physical effects in the brain, including a reduction in hippocampal and mPFC volume7 and increase in activity of default mode network8, though the effect size of such human studies is relatively small. Most drugs for MDD have been developed almost 60 years ago, and their efficacy is usually poor for most people – the effect size of pharmacological interventions is 0.35 compared to 0.2 of placebo9, many patients are completely treatment-resistant and for the patients for whom the drug works, around 70% present a recurrence of symptoms within 6 years.10
The lack of effectiveness of traditional pharmacological treatments has led to exploring the development of many alternative treatments, including psychedelic substances. Ayahuasca has been shown to be effective for managing treatment resistant MDD in a randomized clinical trial.11 Although the main ayahuasca active agents, mainly dimethyltryptamine (DMT) and monoamine oxidase inhibitors are well known, the mechanisms through which they act on depression and other affective disorders remain elusive. The effectiveness of ayahuasca and DMT have been also shown in rodents for anxiogenic behavior12 and non-human primates13 for depressive symptoms, suggesting that this drug may be a powerful tool to understand the underlying neurobiology of depression in the mammalian brain.
Marmosets, the model of choice in this study, present many advantages over other non-human primates: they are small, allowing for a simpler housing infrastructure, they have relatively short life cycle (reaching sexual maturity in 18 months and old age around 8 years)14, their entire genome has been sequenced15, they can express GCaMP with viral vector injections16 and, most relevant for this study, they have a well-established model of depression17, which can be ameliorated with the use of ayahuasca13. Marmosets are a great tool for translational neuroscience because of the deep homology in brain circuitry mediating social behavior and reward, extensive use of vision for social signaling and the fact that these primates present depression-like symptoms in nature.17
The goal of this study is to explore the brain mechanisms underlying: 1) the progression of depressive symptoms and 2) what is the mechanism by which ayahuasca counteracts these symptoms. We will use miniscopes implanted in the vmPFC and field electrodes in the hippocampus – both brain regions previously shown to be associated with depression – and analyze brain activity before social isolation, during social isolation and during treatment (both with an SSRI and with ayahuasca).
Project description
The first thing that needs to be considered for this project is the way in which MDD will be modeled in marmosets. To achieve this, the protocol for social-isolation-induced depression in marmosets, described by Galvão-Coelho et al. (2017)17 will be followed. This protocol is relatively simmple and has been shown to accurately reflect multiple behaviors and physiological changes observed in depressed primates in the wild as well as in humans. It consists of separating juvenile marmosets from their original homecage, where thay are housed in a family context, and housing them alone in a new homecage (this alone appears to be sufficient to induce depression).
In order to study how activity in the vmPFC and the hippocampus changes throughout the progression of depression in the selected model, as well as evaluating the effect ayahuasca treatment has on these physiological changes and the associated behavioral profile; animals will be divided into four treatment groups, containing 6 animals each (ideally 3 males and 3 females, in order to account for intersexual variation): 1) baseline group, that won’t be exposed to social isolation at any point, 2) a negative control group, socially isolated but receiving only saline solution as treatment, 3) a possitive control group, receiving 0.2 mL/100 g of nortriptyline (an anti-depressant with clinical use) and 4) an ayahuasca group, receiving 6.32mg/kg of ayahuasca (dose adjusted for mass and body surface from a human effective dose.11The animals will be moved into isolation (except for the baseline group) after 4 weeks of daily baseline behavioral and physiological measuremnts, performed after complete recovery from surgical procedures. They will be kept on isolation for 8 weeks and measurements will be obtained daily during weeks 1, 2, 7 and 8. On weeks 9 and 10 drug and vehicle treatments will be applied. Measurements will be collected daily during these two weeks, along with the following two weeks (possible delayed effects). The behavioral parameters that will be meassured are: scent marking, individual piloerection, self grooming, scratching, somnolence, feeding (quantity and frequency) and sucrose solution ingestion. The physiological data we aim to obtain on the other hand includes fecal cortisol levels and changes in brain activity on the vmPFC and anterior hippocampus, as meassured by changes in calcium dynamics and electical activity, respectively.
For the purpose of meassuring changes in neuron activity in the vmPFC using calcium imaging, AAVs (Thy1S-tTA; TRE3-GCaMP6f) in solution will be injected into Brodmann area 32, a region of the vmPFC associated with depressive behavior in humans and animals, and that has been proven to have depression-relevant connections to the anterior hippocampus in primates18; this should result in sufficient expression of GCaMP6f and allow us to control said expression through doxycyclin treatment. After 3-4 weeks of incubation time, to ensure stable expression of the introduced constructs a new surgical procedure will be performed, this time to introduce a GRIN lens on area 32 (following the protocol by Kondo et al. (2018)19, as well as introducing the electrodes on the anterior hippocampus and mounting a headstage20 that will help support both measuring devices.After 5-7 weeks of recovery the base of the miniscope will be fixated to the skull, allowing us to securely mount the device on the animals’ heads. Baseline data collection will begin after a further 4-6 weeks for recovery and habituation. 1h of behavioral measurements and 15min of imaging/electrophysiologydata will be collected every day at the same time,in order to avoid circadian variation. The time of physiological recording is limited to 15min in order to produce managaeable amounts of data and to avoid the effects of photobleaching. Additional to this fecal cortisol levels will be meassured on samples collected daily, in order to obtain information about HPA function.
Image and data analysis
Miniscope
In the miniscope analysis pipeline, it is important to perform an image registration in order to correct for movement artefacts. The NoRMCorre library for MATLAB would be preferred since it is a processing-optimized non-linear motion correction, which means that it takes into account that the brain stretches and compresses as the animal moves around. To do cell segmentation for miniscope data, the commonly used analysis is constrained nonnegative matrix factorization optimized for epifluorescent data (CNMF-E), which creates a mathematical model for the background noise and updates it every frame – reducing false positive and false negatives compare to normal CNMF or other methods such as manual ROI definition or PCA/ICA. [1]*
Field electrode
Multi-channel data will be processed according to Mohan et al. (2019)21. Briefly, initial spike sorting will be done using Mclust 4.322 and unit clustering will be performed first semi-automatically using klustakwick23 for initial isolation of units, and then manually, taking into consideration standard parameters such as length of refractory period, spike shape and stability of spiking activity throught the recording. Units can then be clasified based on their spike waveform and analized individually.
Combining miniscope data with electrophysiology data
It is possible to compare miniscope data with electrophysiology data by dimension reduction: essentially, for every cell you capture with the miniscope, the calcium data can be deconvolved and thresholded in order to get unidimensional points of activity. In this way, complex video data would be reduced to the same data time that one would get from single-units in electrophysiology data.[2]* (Figure 1)
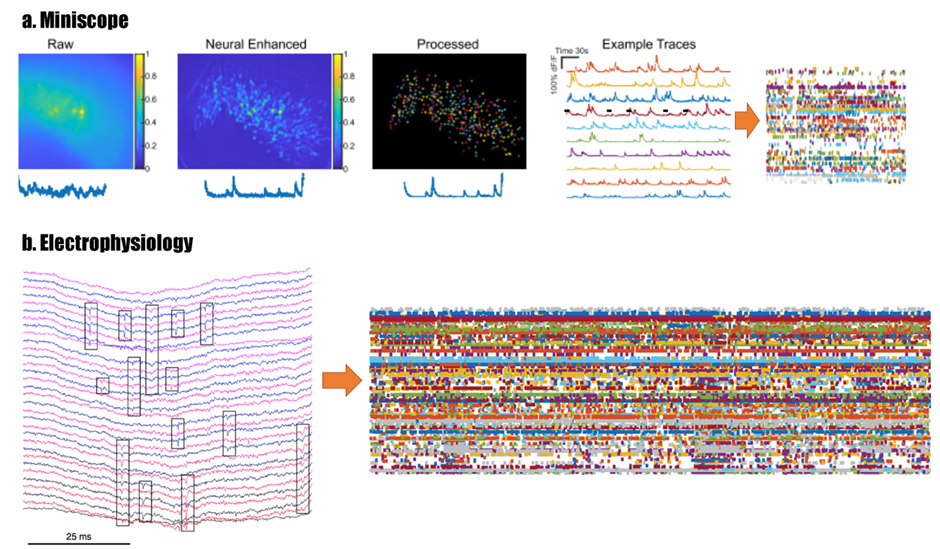
This dataset could be used to compare similar behaviors (when the animal is not moving) over multiple time points (before social isolation, early social isolation, late social isolation, early treatment and late treatment). One could group the data from individual animals (grouping by gender, grouping by treatment type) and assess if there are any commonalities in the development of symptoms of depression and how treatments acts on those patterns of activity. The statistics used will depend on the parameters being assessed – e.g. Mann-Whitney to compare behavioral data (which is not normally distributed), repeated measures ANOVA to assess changes in activity in vmPFC and hippocampus within the same animal, General Linear Models (GLM) Fisher’s post hoc test to assess correlations (activity and behavior).
Whatever changes are found, it would be important to translate the findings both to lower models to ask more mechanistic questions and to humans to potentially come up with new treatments. For example, if it is found that there is a peak of activity in the vmPFC during depression development but it then becomes less active during late stages of social isolation, one could make use of the advanced genetic toolbox for rodents to devise engram-specific/cell-specific studies (e.g. KO of 5-HT2A receptor and assess effectiveness of treatment, fluorescent serotonin probes); but one could also devise a clinical trial in which that brain region is specifically targeted with transcranial magnetic stimulation in patients with familial depression, potentially preventing the progression of symptoms.
It is important to note that the extent of this research project, given the amount of work it requires as well as the time it will take (around 35 weeks, without counting data analysis) goes beyond what could realistically be achieved by a student on a 6 month internship. If necessary, said student could focus only on the social isolation part of the project and make immense contributions to the study.
Relevance of this project
Although the relevance of rodent models for the study of MDD cannot be denied, it is clear that they suffer from limitations that may be limiting their translatability, both in the context of general neuroscientific research (e.g. phylogenetic distance from humans) and in the specific context of depression, such as most models not reflecting behaviors that naturally occur in wild rodents, a limited social structure24 and considerable divergence in serotonin receptor expression in the brain25. Non-human primate models, such as marmosets, animals with closer phylogenetic relation to humans, relatively complex social structures in the wild24 and display of mood disorder-associated behaviors similar to other larger primates including humans, both in captivity and in their natural environment17,26 make valuable candidates for future development of models, which could result in a higher translation potential. In this context, beginning to understand the mechanisms that drive depressive behavior in the marmoset models at cellular and systemic levels, as well as the effect potential treatments have on them, could improve our knowledge of the neurological phenomena that drive this disease, as well as to some extent bridging the gap between the knowledge that has been developed in rodents and the current deficiencies in the clinic.
The increased similarity marmosets have with humans, which gives them their great potential value for neuroscientific research such as ours, also makes them a justified source of ethical concern. In order to minimize suffering and maintain an appropriate life quality, marmosets will be housed in comfortable, enriched home cages, with appropriate access to water and food and with an appropriate number of family members (this last one with the exception of when necessary for the experiments), all in compliance with local and international standards (Brazilian Institute of Environment and Renewable Natural Resources, Animal Behavior Society and the International Primatological Society). Humane endpoints consistent with international guidelines will be set for every experiment and consistent monitoring of suffering and well-being will be performed, in order to detect and avoid excessive suffering. In order to increase the power of this study without an indiscriminate increase in sample size, this study uses a paired design, in which the neuronal activity in the same animal is measured before, during and after social isolation and during treatment. This intergroup design setup will provide the most amount of data per animal, allowing for an unraveling of more subtle differences than what it would be possible with an intragroup design.
References
1. Kessler, R. C. & Bromet, E. J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 34, 119–138 (2013).
2. Li, X. et al. Depression-Like Behavioral Phenotypes by Social and Social Plus Visual Isolation in the Adult Female Macaca fascicularis. PLoS One 8, (2013).
3. Felger, J. C. Role of Inflammation in Depression and Treatment Implications. in Handbook of Experimental Pharmacology 250, 255–286 (Springer New York LLC, 2019).
4. Bansal, Y. & Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 14, 610–618 (2016).
5. Keller, J. et al. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 22, 527–536 (2017).
6. Liang, S., Wu, X., Hu, X., Wang, T. & Jin, F. Recognizing depression from the microbiota–gut–brain axis. International Journal of Molecular Sciences 19, (2018).
7. Drevets, W. C., Price, J. L. & Furey, M. L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 213, 93–118 (2008).
8. Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611 (2015).
9. Cuijpers, P. et al. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: A meta-analysis of direct comparisons. World Psychiatry 12, 137–148 (2013).
10. Verduijn, J. et al. Reconsidering the prognosis of major depressive disorder across diagnostic boundaries: Full recovery is the exception rather than the rule. BMC Med. 15, (2017).
11. Palhano-Fontes, F. et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol. Med. 49, 655–663 (2019).
12. Cameron, L. P., Benson, C. J., Defelice, B. C., Fiehn, O. & Olson, D. E. Chronic, Intermittent Microdoses of the Psychedelic N, N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem. Neurosci. 10, 3261–3270 (2019).
13. Da Silva, F. S. et al. Acute effects of ayahuasca in a juvenile non-human primate model of depression. Brazilian J. Psychiatry 41, 280–288 (2019).
14. Preuss, T. M. Critique of pure marmoset. Brain. Behav. Evol. 93, 92–107 (2019).
15. Worley, K. C. et al. The common marmoset genome provides insight into primate biology and evolution. Nat. Genet. 46, 850–857 (2014).
16. Park, J. E. et al. Generation of transgenic marmosets expressing genetically encoded calcium indicators. Sci. Rep. 6, (2016).
17. Galvão-Coelho, N. L., Galvão, A. C. de M., da Silva, F. S. & de Sousa, M. B. C. Common marmosets: A potential translational animal model of juvenile depression. Front. Psychiatry 8, 1–17 (2017).
18. Wallis, C. U., Cockcroft, G. J., Cardinal, R. N., Roberts, A. C. & Clarke, H. F. Hippocampal Interaction With Area 25, but not Area 32, Regulates Marmoset Approach–Avoidance Behavior. Cereb. Cortex (2019). doi:10.1093/cercor/bhz015
19. Kondo, T. et al. Calcium Transient Dynamics of Neural Ensembles in the Primary Motor Cortex of Naturally Behaving Monkeys. Cell Rep. 24, 2191-2195.e4 (2018).
20. Roy, S. & Wang, X. Wireless multi-channel single unit recording in freely moving and vocalizing primates. J. Neurosci. Methods 203, 28–40 (2012).
21. Mohan, H. et al. Functional Architecture and Encoding of Tactile Sensorimotor Behavior in Rat Posterior Parietal Cortex. J. Neurosci. 39, 7332–7343 (2019).
22. Fraley, C. & Raftery, A. E. Enhanced Model-Based Clustering, Density Estimation, and Discriminant Analysis Software: MCLUST. J. Classif. 20, 263–286 (2003).
23. Harris, K. D., Henze, D. A., Csicsvari, J., Hirase, H. & Buzsáki, G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. Journal of Neurophysiology 84, (2000).
24. Hendrie, C. A. & Pickles, A. R. Depression as an evolutionary adaptation: Implications for the development of preclinical models. Med. Hypotheses 72, 342–347 (2009).
25. Susser, E., Keyes, K. & Mascayano, F. Healthy pregnancy and prevention of psychosis. World Psychiatry 17, 357–358 (2018).
26. Barros, M. & Tomaz, C. Non-human primate models for investigating fear and anxiety. Neuroscience and Biobehavioral Reviews 26, 187–201 (2002).
[1]* In terms of data size, it would not be possible to do whole day recordings, since the data needs to be stored in an SD card and transferred manually to a computer once a week. The timing of 15 minutes once per day would yield enough data to be able to draw inferences about changes in neuronal activity over time and also reduce the amount of GCaMP photobleaching observed. 30 hours of miniscope data would be acquired per week for the 24 animals – since data would not be acquired during the weekends, it would be optimal to have the more processing-demanding part of the analysis running on a powerful computer over the weekend. Another consideration of the miniscope is that we assume it would be possible to record the same neuronal population over multiple days. One main disadvantage of the wireless UCLA miniscope compared to the NiNscope is a lack of focus control in software, so a pilot study would be necessary to assess if daily changes in the focus ring would be necessary.
[2]* It is important to realize that electrophysiology can provide information about many hundreds of single units, while the miniscope only captures about 100 cells. This sample size difference should be taken into account in the statistical analysis, as well as the delay between neuronal activation and increase in GCaMP fluorescence – which would need to be taken into account in the analysis.